This lesson describes the relationship between the structure and function of the giant covalent substances graphite and diamond.
Uses of diamond and graphite in chemistry.
Diamonds so prepared are called synthetic or artificial diamonds.
When you use a pencil sheets are rubbed off and stick to the paper.
Synthetic diamonds are widely used in industry in cutting and grinding tools.
Solid carbon comes in different forms known as allotropes depending on the type of chemical bond.
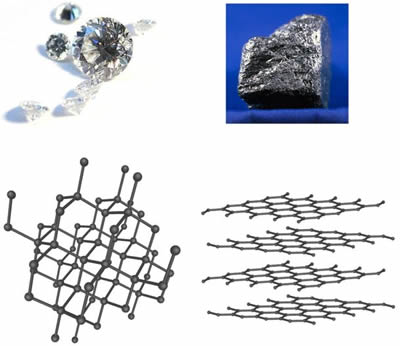
Diamond has a tetrahedral structure where as graphite has an hexagonal arrangement.
Diamonds usually have eight sides forming double pyramids.
Carbon atoms in diamond form a tetrahedral arrangement properties and uses.
Graphite has a giant covalent structure in which.
Diamond and graphite are allot ropes of each other.
These minerals chemically consist of carbon atoms with different physical properties.
Graphite is used as lubricant either as a powder or as a dispersion in oil or water.
Makes diamond useful for cutting tools such as diamond tipped glass cutters and oil rig drills.
Graphene a naturally occurring ingredient in graphite has unique physical properties and is one of the strongest known substances.
This is because of the relatively large amount of space that is wasted between the sheets.
Graphite is mostly used for refractory battery steel expanded graphite brake linings foundry facings and lubricants.
Mixed with clay it is used in lead pencils.
Attractions between solvent molecules.
These minerals in general are known to be as polymorphs having the same type of chemistry but of the various crystalline structures.
The low density 2 26 g cm 3 of graphite is due to large distance between different layers of carbon atoms.
The dispersion of graphite in oil is known as oil dag and in water is known as aqua dag.
Diamond is a solid form of pure carbon with its atoms arranged in a crystal.
Graphite is insoluble in water and organic solvents for the same reason that diamond is insoluble.
Graphite is a stable form of naturally occurring carbon also known as plumbago blacklead or mineral carbon.
Graphite has a lower density than diamond.
The chemical properties of synthetic and natural diamonds are the same.
Some have six sides and they form cubes.
The two most common allotropes of pure carbon are diamond and graphite in graphite the bonds are sp 2 orbital hybrids and the atoms form in planes with each bound to three nearest neighbors 120 degrees apart.
Both are made of carbon atoms entirely.